Qualification & Validation
We have a wide range of expertise within the Pharmaceutical and Medical Device industry and clients will therefore be met by experienced validation consultants.
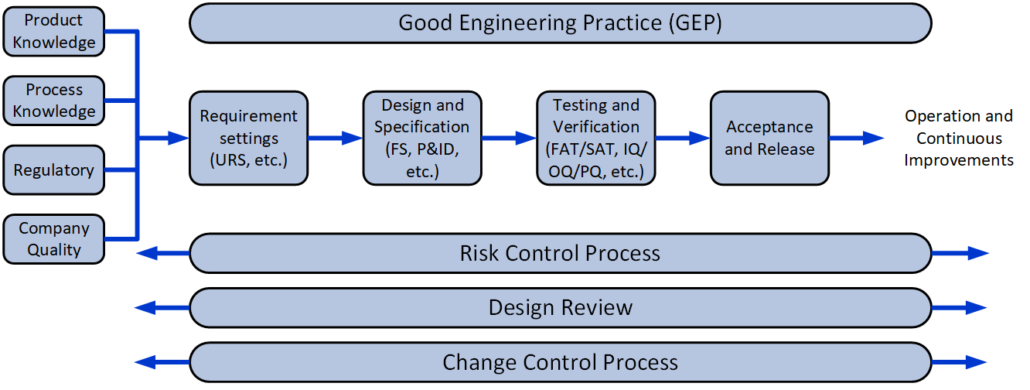
We are experienced within both the traditional V-model and the risk-based approach, which are normally used for the Qualification and Validation projects.
We can help you with
- Requirements-analysis (URS)
- Design, Design-documentation, design review, and design qualification (DQ)
- Risk-analysis – FMEA, FMECA, HACCP, HAZOP, FTA, and PHA
- Validation Plannning
- Change Control
- Commissioning (FAT/SAT)
- Qualification (IQ/OQ/PQ)
- Process validation (PV)
- Cleaning validation (CV)